 By Martin Wellman, Department of Neuroscience, Carleton University
By Martin Wellman, Department of Neuroscience, Carleton University
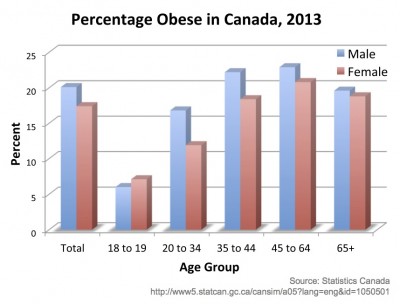
In Statistics Canada’s most recent report on overweight and obesity in 2013, 18.8% of Canadians aged 18 or older were obese based on their body-mass index, while 41.9% of men and 27.7% of women were overweight [1]. We are all aware of these health conditions in Canada and much of the rest of the Western world. Beyond the health factors associated with obesity and overweight, strain is put on the social support system, medical costs rise, and quality of life goes down.
In order to effectively reduce overweight and obesity rates, the issue has to be approached along several fronts
• at the governmental level, through regulation of foods including possibly going so far as banning certain food products or ingredients;
• at the educational level, to increase awareness and promote healthy lifestyles starting at a young age
• at the corporate level, to reduce the use of unhealthy ingredients in products; and
• at the scientific level, to guide governmental policy and educational curricula, as well as to provide an understanding of the physiological systems involved and possibly develop therapeutic agents to aid in weight loss or prevention of weight gain.
 In the field of therapeutic drugs, we have seen very little success, often due to unmanageable side effects. A recent example is that of Rimonanbant (trade name Acomplia), initially approved for use in Europe. In 2008, two years after initial approval, the European Medicines Agency recommended the suspension of marketing for Rimonabant, citing that “the benefits of Acomplia no longer outweigh its risks”, including “an approximate doubling of the risk of psychiatric disorders in obese or overweight patients taking Acomplia compared to those taking placebo”. The agency’s Committee for Medicinal Products for Human Use also stated that “these psychiatric side effects could not be adequately addressed by further risk minimization measures” [2]. The drug was withdrawn the following year [3].
In the field of therapeutic drugs, we have seen very little success, often due to unmanageable side effects. A recent example is that of Rimonanbant (trade name Acomplia), initially approved for use in Europe. In 2008, two years after initial approval, the European Medicines Agency recommended the suspension of marketing for Rimonabant, citing that “the benefits of Acomplia no longer outweigh its risks”, including “an approximate doubling of the risk of psychiatric disorders in obese or overweight patients taking Acomplia compared to those taking placebo”. The agency’s Committee for Medicinal Products for Human Use also stated that “these psychiatric side effects could not be adequately addressed by further risk minimization measures” [2]. The drug was withdrawn the following year [3].
In addition to side effects, the weight-loss associated with Rimonabant (in combination with dieting and exercise), while statistically significant, was certainly not enough to bring an obese individual down to what is considered “normal” weight. Data indicated an approximate 5% reduction in weight following one year of treatment in 50% of participants receiving 20 mg/day [4]. This 5% represents an average drop in weight from 105 kg (231 lbs) to 100 kg (220 lbs). When treatment was extended to two years, the reduced weight compared to placebo controls was maintained, but the extent of the weight loss did not increase. This maintenance, however, was an important result of treatment, as many dieters regain the weight they lose.
As is the case with most conditions, the path to effective pharmaceuticals has been, and likely will be, a long road with minor incremental improvements, and possible setbacks, along the way. The challenge of developing pharmaceuticals with minimal side effects stems in large part from the interactions between various physiological systems. Simply put, there is no bodily function or system that acts in isolation. For the example the use of aspirin as an analgesic may also function in prevention or treatment of certain cardiovascular problems.
 Our research focuses on a particular hormone called ghrelin. Discovered in 1999, ghrelin promotes feeding and is secreted into circulation primarily, but not solely, by the stomach. Ghrelin levels rise when our body detects low energy, particularly before a meal, and decrease once energy supplies increase, such as after a meal. Injection of ghrelin enhances appetite and feeding and promotes storage of fats in both animals and humans. Once activated, ghrelin binds to a receptor called the growth-hormone secretagogue receptor, also known as GHSR1a. It is this receptor that modulates most of ghrelin’s activities. While we have the tools to block this receptor, or to block activation of ghrelin, we know that doing so can have wide-reaching effects beyond reductions in feeding or fat storage.
Our research focuses on a particular hormone called ghrelin. Discovered in 1999, ghrelin promotes feeding and is secreted into circulation primarily, but not solely, by the stomach. Ghrelin levels rise when our body detects low energy, particularly before a meal, and decrease once energy supplies increase, such as after a meal. Injection of ghrelin enhances appetite and feeding and promotes storage of fats in both animals and humans. Once activated, ghrelin binds to a receptor called the growth-hormone secretagogue receptor, also known as GHSR1a. It is this receptor that modulates most of ghrelin’s activities. While we have the tools to block this receptor, or to block activation of ghrelin, we know that doing so can have wide-reaching effects beyond reductions in feeding or fat storage.
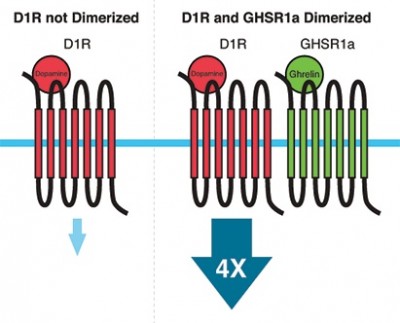
The interaction between the ghrelin system and other biological systems is demonstrated by a fascinating phenomenon called receptor dimerization. Dimerization involves two receptors that come together and physically interact. This physical interaction can change how the receptors work, including increases or decreases in sensitivity, and how long the receptor stays active. In some cases, the dimerized complex has properties entirely different from the two receptors it is composed of. GHSR1a can form dimers with many other receptors. One such dimer involves partnering GHSR1a with the dopamine 1 receptor (D1R), which is heavily involved in reward mechanisms. This includes the rewarding feelings associated with such things as eating or sex. When dimerized with GHSR1a, D1R signaling is amplified four-fold. If we administer weight-loss drugs that inhibit GHSR1a, in theory this should also inhibit signaling through D1R, which would reduce our ability to experience pleasure. When this reduction is severe enough, we call it anhedonia, which is associated with psychiatric conditions such as depression. While in most people this reduction in reward may not be so severe, there may be some who are more prone to such negative consequences.
 In addition to dopamine’s rewarding effect, it is also capable of making us less hungry. In this case, it is the dopamine 2 receptor (D2R) that is responsible, but only when it is dimerized with GHSR1a. Given this, inhibition of GHSR1a should reduce dopamine’s ability to decrease appetite, which is the opposite to what we would normally expect to happen when GHSR1a is inhibited. An interaction between GHSR1a and other neurochemical systems has also been found, including serotonin (associated with depression and schizophrenia).
In addition to dopamine’s rewarding effect, it is also capable of making us less hungry. In this case, it is the dopamine 2 receptor (D2R) that is responsible, but only when it is dimerized with GHSR1a. Given this, inhibition of GHSR1a should reduce dopamine’s ability to decrease appetite, which is the opposite to what we would normally expect to happen when GHSR1a is inhibited. An interaction between GHSR1a and other neurochemical systems has also been found, including serotonin (associated with depression and schizophrenia).
This complexity is a reflection of the tight interconnections between various physiological pathways. This is why drug design that achieves a specific purpose without negative side effects is such a challenge. This process of dimerization extends beyond the ghrelin system, and is part of the intricate knowledge needed for the development of drug treatments. This is not, however, a reason to give up hope in terms of pharmaceuticals. As scientists, our primary goal is to understand how these systems work. The more knowledge we gather, the closer we get to more effective treatments.
Based on
Wellman, M. and A. Abizaid (2015). “Growth Hormone Secretagogue Receptor Dimers: A New Pharmacological Target.” eNeuro 2(2). http://eneuro.org/content/2/2/ENEURO.0053-14.2015.abstract
- Statistics Canada. Overweight and obese adults (self-reported), 2013. Statistics Canada Catalogue no. 82-625-X 2013 [cited 2015 May 13]; Available from: http://www.statcan.gc.ca/pub/82-625-x/2014001/article/14021-eng.htm.
- European Medicines Agency. Press Release: The European Medicines Agency recommends suspension of the marketing authorisation of Acomplia. 2008 [cited 2015 May 13]; Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2009/11/WC500014774.pdf.
- European Medicines Agency. Public statement on Acomplia (Rimonabant): Withdrawal of the marketing authorisation in the European Union. 2009 [cited 2015 May 13]; Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Public_statement/2009/11/WC500012189.pdf.
- Pi-Sunyer, F., et al., Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: Rio-north america: a randomized controlled trial. JAMA, 2006. 295(7): p. 761-775.
Top image courtesy of Mister GC at FreeDigitalPhotos.net
Ice cream image courtesy of stockimages at FreeDigitalPhotos.net