 By Nadine Frost, Department of Chemistry, Carleton University
By Nadine Frost, Department of Chemistry, Carleton University
What did you eat today?
Chances are, whatever it is you chose to eat, you might have also consumed some mycotoxins in your breakfast, lunch, and dinner. Mycotoxins are toxic secondary metabolites produced by fungi that can contaminate many of the world’s major food crops, and are still present in your food even after cooking and processing. Foods such as cereal, bread, coffee, and even beer and wine can be contaminated with mycotoxins such as aflatoxins, fumonisins, and ochratoxins. Once consumed, these compounds have been shown to result in a huge range of health concerns for humans and animals, ranging from carcinogenesis, immunosuppression, teratogenicity (causing birth defects), growth supression, neurological and cardiovascular effects – the list goes on.
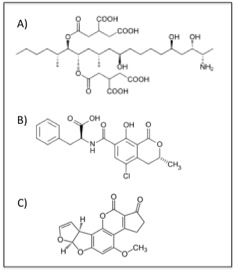
The chemical structures of some of the major mycotoxins. a) fumonisin B1, b) ochratoxin A, and c) aflatoxin B1
Like most people, I don’t like hearing the words ‘toxic’ and ‘food’ in the same sentence. Before you get too worried, remember that for any toxin, “the dose makes the poison”. Living in Ottawa and eating groceries from the Metro by my house, I can rest assured that the products I buy have been tested to ensure that they do not contain toxic levels of mycotoxins. Because these are unavoidable natural contaminants, health agencies including Health Canada establish maximum limits that assure the safety of the food. Testing crops and food products to ensure safety is a huge undertaking. In the developed world, mycotoxin testing is often done in advanced laboratories with expensive equipment. The developing world is often not as fortunate, as political, economic, and social barriers prevent adequate testing and regulation of crops, resulting in dramatic effects on human health.
What if, instead of sending samples away to regulatory agencies, you had a cheaper and more accessible test kit that could be brought to the field to directly screen for the levels of contamination? Our ‘weapon of choice’ to detect these mycotoxins is nothing more than a short piece of DNA, also known as an aptamer. Unlike genomic DNA, aptamers are synthetic and single-stranded, allowing them the freedom to fold into a unique three-dimensional shape. We select for an aptamer that will recognize and bind to a specific mycotoxin, by folding up into a shape that ‘fits’ the chemical structure of the mycotoxin.
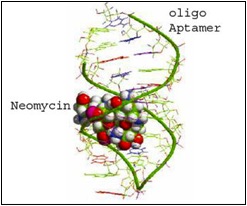
Aptamer binding to neomycin, showing how the target molecule fits into the shape formed by the aptamer.
Source: http://www.genelink.com/newsite/
products/aptamers.asp
Before we go too far, there is a lot of work that has to be done to evaluate how well the aptamer binds to the mycotoxin. We determine the quality of an aptamer based on its binding affinity. In our lab, we worked to develop a new way to screen the binding affinity of aptamers for one of the mycotoxins I mentioned earlier, fumonsin B1, which is found mostly on corn. Essentially, we measure how the enzymatic digestion of the aptamer changes when it is bound to the mycotoxin. This allows us to get a better picture of where in the aptamer the target is binding, and how strong the affinity is.
What we do next is chemically modify the aptamers so that when they bind to the toxin, a signal is generated. This is a sensor – the more toxin present, the stronger the signal. In our lab, we have been working on generating sensors that use fluorescence as the signal. One of the tests that we have been working to develop is measuring another mycotoxin, ochratoxin A, which is mostly found on wheat. Essentially, we link together two very small particles onto the aptamers; one produces fluorescence, the other quenches it. When ochratoxin A is in a sample, it breaks the linkage and allows the fluorescence signal to ‘turn on’. Again, more toxin gives a brighter fluorescence. This testing system has been adapted so that you can perform it on a simple piece of paper and have results within a minute. Simple, portable, and cost effective.
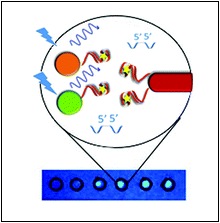
This figure shows how the fluorescence test for ochratoxin A works. The green and orange circles are the fluorescence producers, and the red rod is the fluorescence quencher. They are linked with the aptamer, that binds to ochratoxin. As shown on the paper below, when ochratoxin A is present in the sample, the spot on the paper glows under a UV light.
Working in the lab, it’s easy to forget that the project you’re doing is just one of many ways to approach the problem at hand. Last June, I had the unique opportunity to attend and present my work at the Gordon Research Seminar and Conference on Mycotoxins and Phycotoxins in Easton, MA. There, I learned about the multitude of ways that mycotoxin production, contamination, and regulation are being addressed around the world, which was an eye-opening experience. So, the next time you sit down to enjoy a bowl of cereal, a glass of wine, or a bar of chocolate, take a moment to think of what went into making your food safe to eat.
References:
Frost NR, McKeague M, Falcioni D, DeRosa MC (2015) An in solution assay for interrogation of affinity and rational minimer design for small molecule-binding aptamers. Analyst, 140:6643-51.
Velu R, Frost N, DeRosa MC (2015) Linkage inversion assembled nano-aptasensors (LIANAs) for turn-on fluorescence detection. Chem Commun, 51:14346-9.
Top image courtesy of rakratchada torsap at FreeDigitalPhotos.net