 By Natalie Linklater, Dept. of Civil and Environmental Engineering, Carleton University
By Natalie Linklater, Dept. of Civil and Environmental Engineering, Carleton University
Wastewater; it’s the term we use to denote anything and everything that gets flushed in toilets, rinsed down drains and washed off our streets. It can include residue from soaps and creams, residual pharmaceuticals, pollution from streets, and yes… poop. Tucked away near the banks of rivers and lakes, treatment facilities have a number of steps that aim to clean wastewater before returning it back to the environment.
Wait?! What?!
You read that right. The ultimate goal is to return treated wastewater back into the environment. I know this may come as a bit of a shock but remember that the water cycle teaches us that “all the water on earth today, every drop, is all the water there has ever been on the planet” (National Science Foundation, 2013). To keep our natural waters flowing and drinking water sources clean, treated wastewater needs to be returned back to the environment. There is no secret holding space or second magic flush. It all has to go somewhere, but before it does, we want to make sure it is as clean as possible. That’s why engineers and researchers are continuously re-examining and re-imagining every step of the treatment process to try and find new perspectives to the age old problem: what to do with all this poop!

Water treatment plant
Disinfection is the final stage of the wastewater treatment and is important because it reduces disease causing microorganisms to safe levels before wastewater is released into the wild. The ideal disinfectant is one that would accomplish the required amount of microorganism reduction while being easy to apply, inexpensive, leave little to no chemical residual and have little to no interaction with organic matter, which is abundant in natural and waste waters. This is one tall order! Of the disinfectants that are used today, chlorine is by far the most popular in North America.
Remember I said that engineers and researchers are continuously trying to re-imagine treatment processes? Now, imagine a disinfection process that uses no chemicals but is still able to reduce microbes to below target levels. Ultraviolet disinfection is just such a process. The same type of light that radiates from the sun to cause sunburns can also be used to cause damage to microbial DNA rendering them inactive. In practice, we place UV light bulbs into large tanks and turn on the light for a set amount of time.
The difficulty arises during heavy rain storms. In these instances, extra water from the rain gets funnelled to treatment plants and risks flooding tanks and taking the entire treatment process offline. To prevent this, treatment plants make adjustments to accommodate larger volumes but do so by sacrificing a certain amount of quality. To assure that treatment plants still meet required targets, my research looks at supplementing UV with a secondary disinfectant such as ferrate, peracetic acid or hydrogen peroxide. You may have noticed that these are not your typical disinfectants. These chemicals are all chlorine-free and leave little to no residuals. As an added bonus, using two disinfectants has been shown to have synergistic effects.
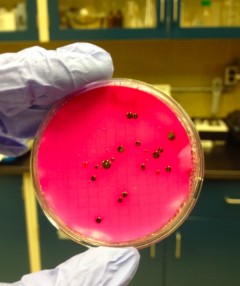
E.coli sample
For the purpose of this blog post let’s discuss results obtained with ferrate, which are very promising! I’ve examined the use of UV and ferrate both individually and in sequence using a number of different parameters. First, UV light in combination with ferrate reduced E.coli bacteria by an additional 80% compared to the use of UV light alone. Using a molecular stain to examine live and dead bacteria after treatment, ferrate also considerably decreased the number of living bacteria. This means that ferrate not only acts on E.coli, but is also effective at reducing a broader spectrum of bacteria. While I was looking at samples under the microscope I observed clumps of live and dead bacteria, which was unexpected.
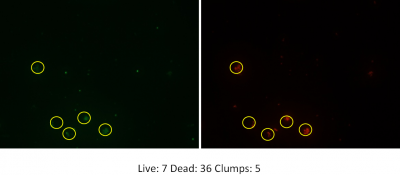
Clumps of live and dead bacteria
This lead to questions such as: what are these clumps, why are they forming and are they a bad thing? The addition of ferrate to wastewater also increased the cloudiness or murkiness of the wastewater. This is because ferrate acts as a coagulant, or a substance that encourages smaller particles in wastewater to agglomerate into larger ones. During the process, bacteria and even larger pollution molecules can get trapped into these larger particles. Thankfully, this clumping isn’t necessarily a bad thing. Larger particles can be removed from wastewater by allowing gravity and time to pull these larger and heavier particles to the bottom of tanks taking trapped bacteria and pollutants with them. So, while wastewater may need to spend more time in the treatment facility to allow for this settling to take place, it means a cleaner wastewater in the end.
 The take home message here is that wastewater treatment is important to maintain the health of our beaches, rivers, lakes and drinking water sources. UV is an effective non-chemical and chlorine-free method for wastewater disinfection. Secondary disinfectants such as ferrate have the potential to offer treatment plants the operational flexibility they need to maintain the highest treatment standards.
The take home message here is that wastewater treatment is important to maintain the health of our beaches, rivers, lakes and drinking water sources. UV is an effective non-chemical and chlorine-free method for wastewater disinfection. Secondary disinfectants such as ferrate have the potential to offer treatment plants the operational flexibility they need to maintain the highest treatment standards.
Based on a paper presented by Linklater, N. & Örmeci, B. at the 29th Eastern Canadian Symposium on Water Quality Research (2015).
References
National Science Foundation, (2013). The Water Cycle. [online] Available at: https://www.youtube.com/watch?v=al-do-HGuIk [Accessed 19 Aug. 2015].