Our group is mainly interested in the development of precursors and processes for atomic layer deposition (ALD); we were the first academic research group in Canada to work in this field.
As synthetic chemists, we try to determine the mechanism of the surface chemistry and thermal decomposition routes to better design precursors and tune our processes. We look at many different processes and target films, but some themes can broadly be drawn through our work.
Stepwise Mechanistic Chemistry
Mechanistic understanding allows a significant advantage to an ALD process: knowing how thermolysis occurs permits redesign of precursors or processes to exploit the mechanism to tune processes.
The standard mechanistic steps in metal-organic chemistry can illuminate thermal chemistry and surface chemistry. Our group has published a perspective article on this topic, and have used this understanding to detail surface and thermal chemistry, for instance, in Co metal and MoN deposition chemistry.
Group 11 Compounds
We have been interested in group 11 metals for several years, and have contributed to precursor and process design for copper and gold metal ALD solutions. 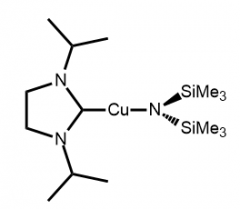
We employ guanidinates, iminopyrrolidinates, and silylamides as anionic ligands for copper metal precursors, occasionally employing carbenes as a neutral coordination ligand to complete the coordination environment for Cu(I). The best example of a precursor is a hexamethyldisilazido N-heterocycliccarbene copper(I) that deposits using a thermal process on a noble metal or with plasma hydrogen on silicon and glass.
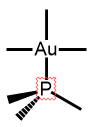 We have also reported the first ALD deposition of gold metal using plasma oxygen and water, with followup examples of hydrogen plasma and ozone processes. These processes use a trimethyl trimethylphosphine gold(III) precursor that is a liquid at room temperature and exhibits surprising stability to oxygen and water.
We have also reported the first ALD deposition of gold metal using plasma oxygen and water, with followup examples of hydrogen plasma and ozone processes. These processes use a trimethyl trimethylphosphine gold(III) precursor that is a liquid at room temperature and exhibits surprising stability to oxygen and water.
Chemistry of ALD – A Textbook
You can buy it here, and the Amazon author page can be found here. I hope it to be the backbone of a course on ALD chemistry; it isn’t comprehensive with respect to precursors or surface chemistry but rather goes through the core concepts of understanding ALD from a chemistry point of view. Barry, S. T. Chemistry of Atomic Layer Deposition; De Gruyter. |
Ligand DesignWe developed iminopyrrolidinate ligands as an answer to the low-temperature thermolysis that can occur in amidinates and guanidinates. This took a LOT of thermolysis, modeling, and grad-student-hours. It might be my favourite chemistry we’ve done. The key message is:
Barry, S. T. Amidinates, Guanidinates and Iminopyrrolidinates: Understanding Precursor Thermolysis to Design a Better Ligand. Coord. Chem. Rev. 2013, 257, 3192 – 3201. |
Precursors for CVD and ALDIn general, our group stays up at night worrying about five things:
We want to control these in all of our precursors. The key messages are:
Koponen, S. E.; Gordon, P. G.; Barry, S. T. Principles of Precursor Design for Vapour Deposition Methods. Polyhedron 2016, 108, 59 – 66. |
Funding
 Canadian Foundation for Innovation |
 Carleton CarletonUniversity |
 Ontario OntarioResearch Fund |
 Royal Canadian Royal CanadianMint |
 Vinnova Vinnova |
 NSERC NSERC |
 Solvay Solvay |
 Lam Lam |

