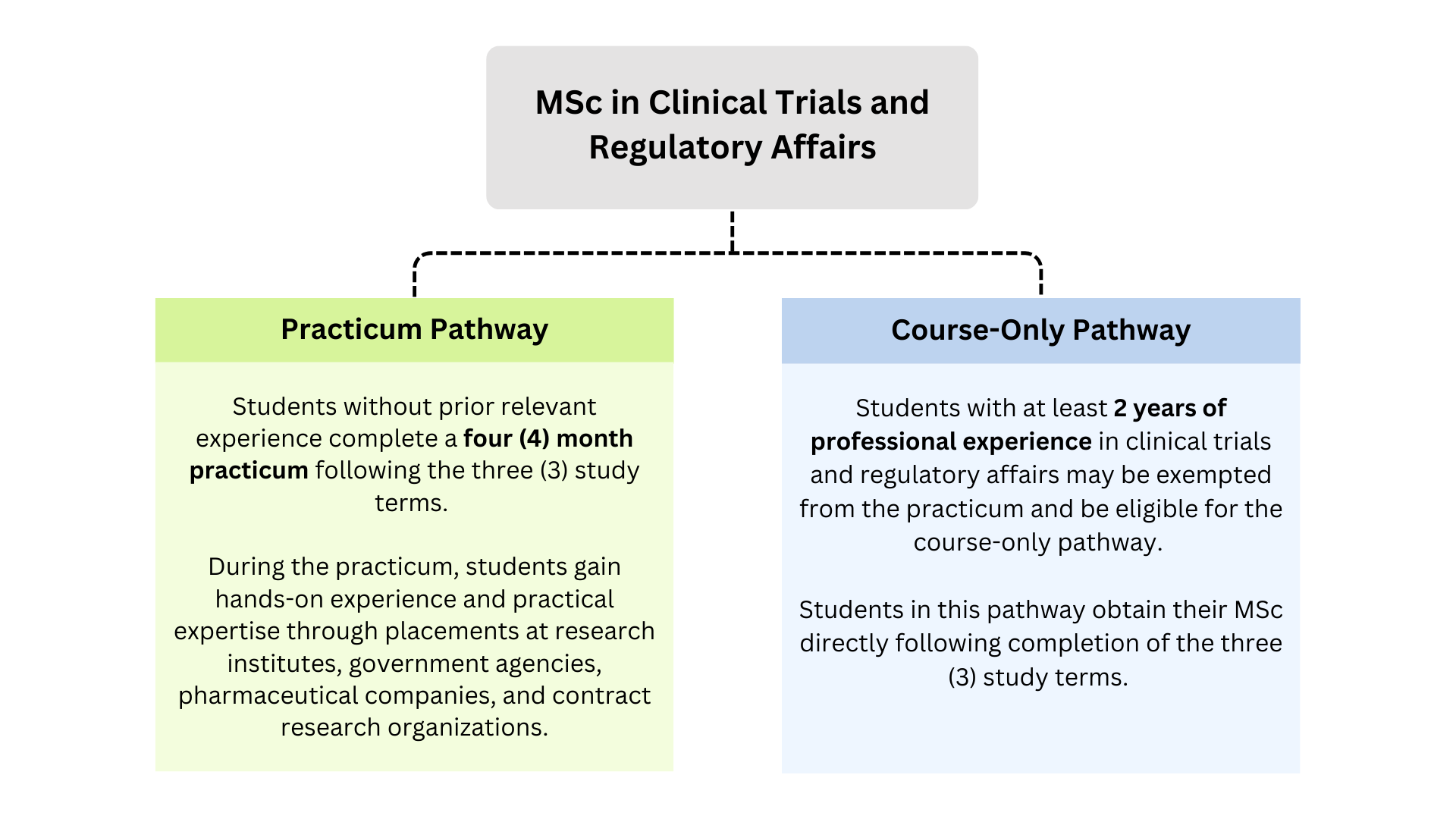
Program Pathways
Full-Time Studies
The MSc program consists of three (3) consecutive study terms and a four (4) month practicum consisting of a placement at research institutes, government agencies, pharmaceutical companies, and contract research organizations (CRO). The program is completed over 16 months of full-time studies.
Note: Students with at least two (2) years of relevant experience in clinical trials and regulatory affairs may be exempted from the practicum and be eligible to complete the course-only pathway. If the applicant has had some experience in clinical trials and regulatory affairs previously, at least one letter of reference from a supervisor is preferred.
Snapshot of program layout
Below is a snapshot of the program layout:
- Term 0 consists of two (2) 0-credit primer courses to be completed online in the two (2) weeks prior to Term 1. These primer courses are designed to equip students with basic skills and knowledge about clinical trials, regulatory requirements, ethics, and data requirements to ensure that students are well prepared to excel in their studies.
- Terms 1, 2, and 3 consist of three (3) 0.5-credit courses each and provide students with the knowledge and skills to design, conduct, manage and evaluate clinical trials across healthcare environments.
- For students in the practicum pathway, Term 4 consists of a four (4) month placement in a health science environment, allowing students to acquire hands-on experience and practical expertise.
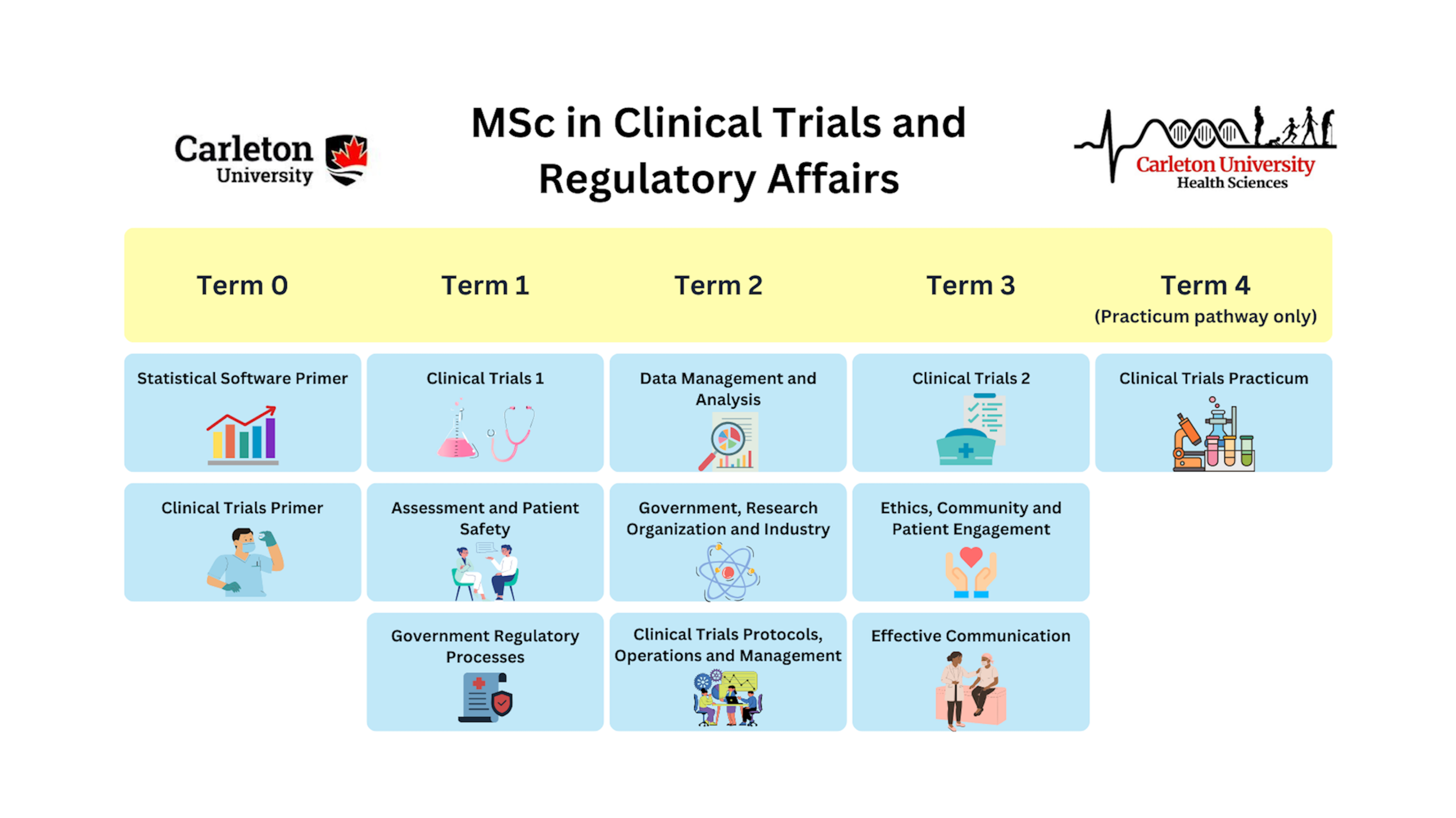
Required Courses
Term 0 (0-credit): online modules to be completed in the 2 weeks prior to Term 1
- HLTH 5101: Statistical Software and its Application to Health Sciences Primer
This is a 0-credit course. Introduction to statistical software used to analyze health research data. Data management topics include data entry, manipulation, and elementary statistical analyses using SAS, SPSS, Stata and R. Other topics include privacy/maintaining security of health datasets. For students without strong backgrounds in biostatistics/data handling.
Includes: Experiential Learning Activity
- HLTH 5811: Clinical Trials Primer
This is a 0-credit course. Overview of the vast area of clinical trials of drugs and devices, and principles of informed consent, regulatory requirements, rigorous documentation, analysis, and reporting. Students will also work on certificates in biomedical ethics, good clinical practice, and others, for example from CITI Canada.
Term 1 (1.5 credits)
- HLTH 5812: Clinical Trials 1 – Introduction
This is a 0.5 credit course. Fundamentals of trials of health products and different phases and types of clinical trials. Investigator vs. sponsor-initiated trials, different regulatory agencies, the use of randomization, blinding, registration regulatory requirements, rigorous documentation, and common trials.
- HLTH 5814: Assessment and Patient Safety for Clinical Trials
This is a 0.5 credit course. The importance of efficacy and safety measurements, biosamples, pharmacokinetics, pharmacodynamics, drug mechanism of action, reporting of harm, Data and Safety Monitoring Board, pharmacovigilance, and consideration of special populations. Good clinical practice, good medical practice, and good laboratory practice.
Includes: Experiential Learning Activity
- HLTH 5816: Government Regulatory Processes
This is a 0.5 credit course. Regulatory agencies (Health Canada, US Food and Drug Administration, European Medicines Agency) will be compared. Harmonization efforts of national drug approval agencies, timelines for an investigational New Drug Application including labeling, accelerated approval, breakthrough designation, orphan drugs, and biologics licence application.
Term 2 (1.5 credits)
- HLTH 5815: Principles of Data Management and Analysis in Clinical Trials
This is a 0.5-credit course. Randomization, biomarkers, endpoints, estimands, sample size requirements, random error and bias, multiple testing correction, intent-to-treat versus per-protocol, equipoise and stopping rules for trials, database development, validation and reporting/transferring, development of statistical analysis plans, and considerations around missing data.
- HLTH 5817: Government, Research Organizations and Industry
This is a 0.5-credit course. Overview of regulatory requirements of pharmaceutical companies, contract research organizations, and communication with regulatory agencies. Negotiation and collaboration between sectors, incentives such as FDA priority review vouchers, project management, manufacturing and distribution, phase IV post-marketing and continued monitoring, pharmacovigilance, and post-marketing changes.
- HLTH 5819: Clinical Trials Protocols, Operations and Management
This is a 0.5-credit course. Clinical protocols, electronic case report forms and guidelines, data management plan, monitoring plan, pharmacy manual, standard operating procedures, manual of operating procedures, delegation of authority logs and training logs. Leadership, logistics, and budgeting.
Term 3 (1.5 credits)
- HLTH 5813: Clinical Trials 2
This is a 0.5-credit course. Other trial designs, recruitment of patients, data collection and quality control, interim monitoring, audits, inspections, and timelines. Includes a four to six-week placement at a clinical or regulatory site, contract research organization (CRO), or similar institution involved in clinical trials.
Includes: Experiential Learning Activity
- HLTH 5818: Ethics, Community and Patient Engagement
This is a 0.5-credit course. Patient engagement, equipoise, informed consent, ethics board, monitoring, reporting/release of data in the literature, compassionate/expanded access, patient foundations, liaisons and advocates. Engaging with Indigenous communities and special populations. Considerations around translation research, genetics, biosimilars, and labeling.
- HLTH 5821: Effective Communication in Clinical Research and Regulatory Environments
This is a 0.5-credit course. This course equips students with the skills to convey complex information in decision-based discussion and explain complex regulatory environments, preparing them for impactful roles in clinical research.
Note: this course may be substituted for another 0.5-credit elective science course (to be determined).
Term 4: Practicum pathway (1.5 credits)
- HLTH 5820: Clinical Trials Practicum
This is a 1.5 capstone credit course required for students in the practicum pathway only. This course involves experiential learning at a clinical site, regulatory site, contract research organization (CRO), or similar institution involved in clinical trials. Students will demonstrate the knowledge and skills gained and will present on their experience, efforts, and lessons learned.
Includes: Experiential Learning Activity
Part-Time Studies
The program is completed over 28 months of part-time studies.
Required Courses
The typical course sequence for part-time students is as follows:
Term 1:
- HLTH 5101: Statistical Software and its Application to Health Sciences Primer (Primer)
- HLTH 5811: Clinical Trials Primer (Primer)
- HLTH 5812: Clinical Trials 1: Introduction
- HLTH 5816: Government Regulatory Processes.
Term 2:
- HLTH 5815: Principles of Data Management and Analysis in Clinical Trials
Term 3:
- HLTH 5813: Clinical Trials 2 (includes 6-week placement)
- HLTH 5821: Effective Communication in Clinical Research and Regulatory Environments
Term 4:
- HLTH 5814: Assessment and Patient Safety for Clinical Trials
Term 5:
- HLTH 5817: Government, Research Organizations and Industry”
- HLTH 5819: Clinical Trials Protocols, Operations and Management
Term 6:
- HLTH 5818: Ethics, Community and Patient Engagement
Term 7:
- HLTH 5820: Clinical Trials Practicum
For any advising questions please contact clinicaltrials@cunet.carleton.ca.
Share: Twitter, Facebook
Short URL:
https://carleton.ca/healthsciences/?p=5128

