Past Event! Note: this event has already taken place.
2021-22 Health Sciences Seminar Series – Dr. Dean Smith
January 20, 2022 at 12:00 PM to 1:00 PM
| Location: | This is a virtual event and will take place over Zoom (registration button below). |
The Department of Health Sciences is pleased to host the next seminar in our series.
Presenter: Dr. Dean Smith, Associate Director at the Center for Biologics Evaluation and the Advisor to the Director General at Biologics and Radiopharmaceutical Drugs Directorate for Health Canada
Key Scientific and Regulatory Principles That Support Expedited Authorization of COVID-19 Vaccines
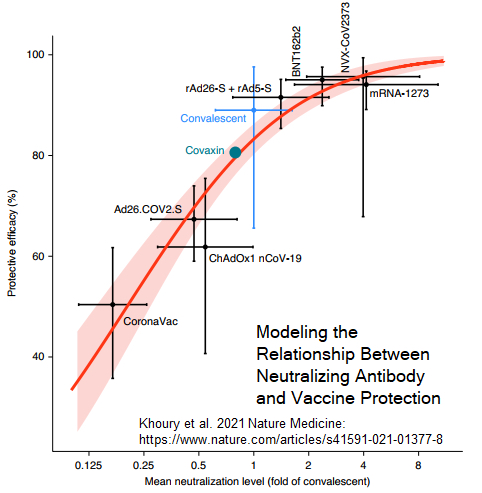
It is essential for regulatory authorities to support innovation through appropriate and efficient risk/benefit driven review pathways and to come to timely evidence-based decisions. The current COVID-19 pandemic highlights the importance of Health Canada’s (HC) regulatory lessons learned over the past twenty years. These lessons have drawn from our regular vaccine review activities, preparedness efforts related to bioterrorist threat vaccines, and earlier pandemics (2003 SARS-CoV-1, 2009 H1N1, 2014/2016 large-scale Ebola outbreaks in West Africa). As of a result of the above experiences, and prior to the current pandemic, HC embarked on regulatory renewal to provide an agile regulatory framework to support ongoing regular, as well as emergency, review and authorization activities.
The importance of early phase 1 – 2 dose ranging studies with well-characterized immunogenicity studies to expedite authorization will be highlighted. How this approach also supports the urgently required manufacturing scale-up and related regulatory approvals will be described. Additionally, when combined with phase 3 efficacy and disease break-through data, dose ranging studies permit correlates of protection (CoP) analyses and new vaccine development. Current thinking on CoP and immunobridging options for new COVID-19 vaccine authorizations, evaluation of post-market vaccine effectiveness studies against variants of concern (VOC) and insights into COVID-19 vaccine pharmacovigilance will also be presented.