Pushing the Boundaries of Photoredox Catalysis
Photoredox catalysis is leading to major breakthroughs in synthetic organic chemistry with good functional group tolerance, broad scope and many unprecedented transformations. In particular
pharmaceutical chemists have a strong interest in these methods for generating compound libraries,
late-stage transformations and medium-scale synthesis via continuous flow photoreactors.
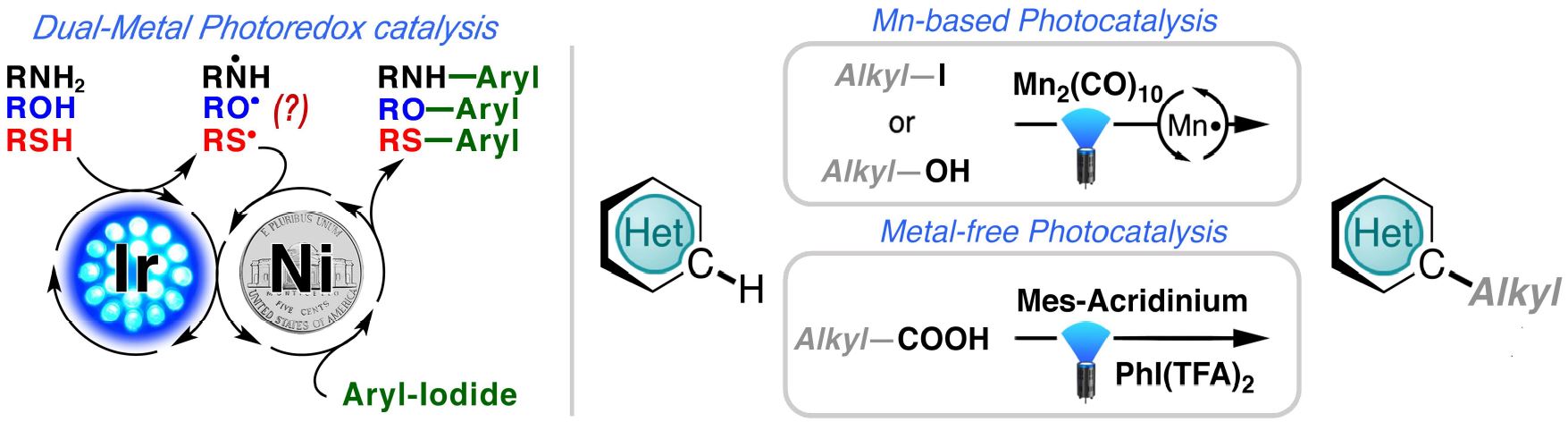
Amongst many active academic players, Prof. David MacMillan and his group reported an
impressive cross-coupling of aryl halides and alcohols using a dual-metal iridium photocatalyst
and a nickel co-catalyst (Nature, 2015, 525, 330).

Using similar Ir/Ni-based photoredox reactions,
our group and AstraZeneca collaborators reported the cross-coupling of thiols and aryl iodides (J.
Am. Chem. Soc., 2016, 138, 1760) and subsequently, C-N bond forming reactions (Angew. Chem.
Int. Ed., 2016, 128, 13413). Recently, in collaboration with researchers at Pfizer, we reported two
light-efficient sp2-sp3 C-C bond forming reactions (Angew. Chem. Int. Ed., 2017, 56, 15309; Org.
Lett., 2018, 20, 3229).
Despite a growing number of photoredox reactions being reported, concrete evidence for their
complex mechanisms and intermediates is often lacking. Using the mechanistic knowledge from
our previous studies, we applied DFT and kinetics methods for the selection of unusual photoredox
substrates. As a proof of concept, we implemented novel C-C bond forming reactions using the CH
activation of hydrocarbons and couple them to electron-rich alkenes. Our mechanistic services
in the conversion of alkyl amines to amides via C-H activation using iron-based catalysis will
also be presented.